Question help_outline Image Transcriptionclose Draw the Lewis structure for a peroxide (0,) ion C ?On the Lewis dot structure it shows each O has 6 electrons and that it has a single bond between the two Oxygen atoms To me, each O atom would have 4This is the Lewisdot structure of hydrogen peroxide Here you have two oxygen atoms and the overall required electrons are 6*22=14,An atom of an element is three times as heavier as the mass of an atom of carbon12 Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO −It is an unstable structural isomer of
4 General Organic And Biochemistry 8 E Bettelheim
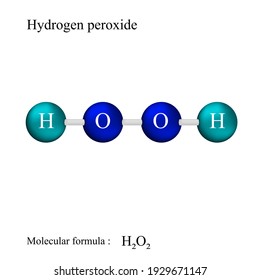
Hydrogen peroxide lewis dot structure
Hydrogen peroxide lewis dot structure-Thus the symmetrical Lewis structure on the left is predicted to be more stable, and it is, in fact, the structure observed experimentally We could use a lone pair on either O or Cl Hydrogen Peroxide The chemical name for H 2 O 2 is hydrogen peroxideChlorine peroxide (also known as dichlorine dioxide or ClO dimer) is a molecular compound with formula ClOOCl Chemically, it is a dimer of the chlorine monoxide radical (ClO·) It is important in
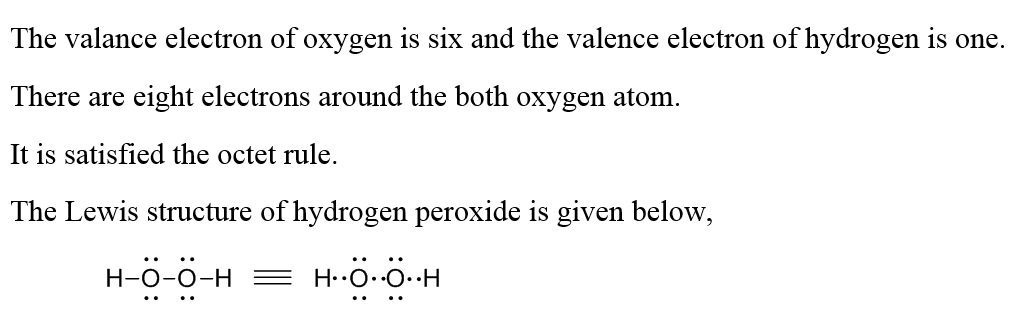


Answered A Construct A Lewis Structure For Bartleby
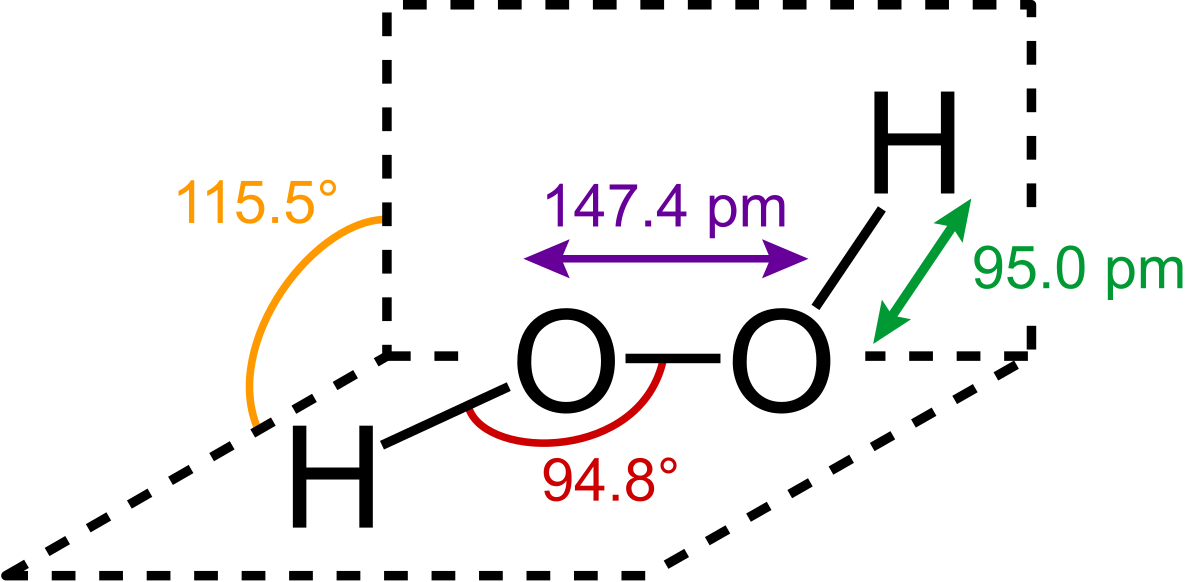
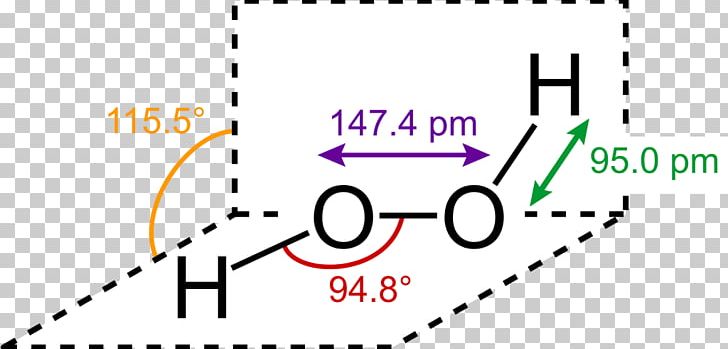
Oct 19, 18 · Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign Toothpastes containing sodium hydrogen carbonate (sodium bicarbonate) and hydrogen peroxide are widely used Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriateMay 03, 19 · The structure of hydrogen peroxide is nonplanar H 2 O 2 has an open book structure with O – O spins The dihedral angle is 111° The OO bond length is 1458 pm and the OH bond length is 9 pm (which is equal to 9 × 10 13 m)Structure & Reactivity in Chemistry Introduction to Molecules IM11 Controversial Lewis structures There is sometimes controversy in science Argument can be an important part of how we arrive at a better understanding of things In the context of molecules and structures, there have been longrunning controversies about how to draw certain
Practice Quiz Lewis StructuresJun 10, 18 · The chemical name for H2 O2 is hydrogen peroxide Its Lewis structure shows us where the valence electrons are located in the molecule, which can aid us in Count the number of electrons, add single bonds between the atoms, using two electrons per bond, arrange the remaining electrons around theSome Lewis structures for peroxides are shown at the right The diagram for O 2 is inadequate, but does show the two unpaired electrons The O ion does not exist in aqueous solution, and neither do the O 2 or O 2 ions, but they do exist in crystals
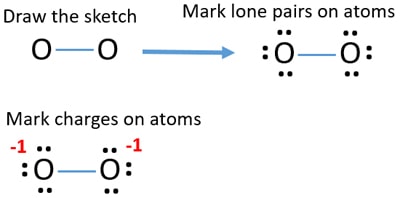
Transcript This is the O2 2 Lewis structure For the peroxide ion, Oxygen has six valence electrons We have two Oxygens, and then we need to take in account these extra two valence electrons up here, so we'll just add them for a total of 14 valence electrons for the 02 2 Lewis structurePotassium peroxide appears as a yellow granular solid Mixtures of potassium peroxide and combustible material readily ignited by friction, heat or contact with moisture Prolonged exposure to fire or heat may cause vigorous decomposition ofO 2 2(peroxide ion) Lewis Structure O 2 2(peroxide ion) anion contains only two oxygen atoms Peroxide anion has 2 charge In O 2 2lewis structure, each oxygen atom has 1 charge and three lone pairs Both oxygen atoms are joint through a single bond In this tutorial, we are going to draw the lewis structure of O 2 2ion step by step



What Is The Electron Dot Structure Of Math Ko 2 Math Potassium Superoxide Quora
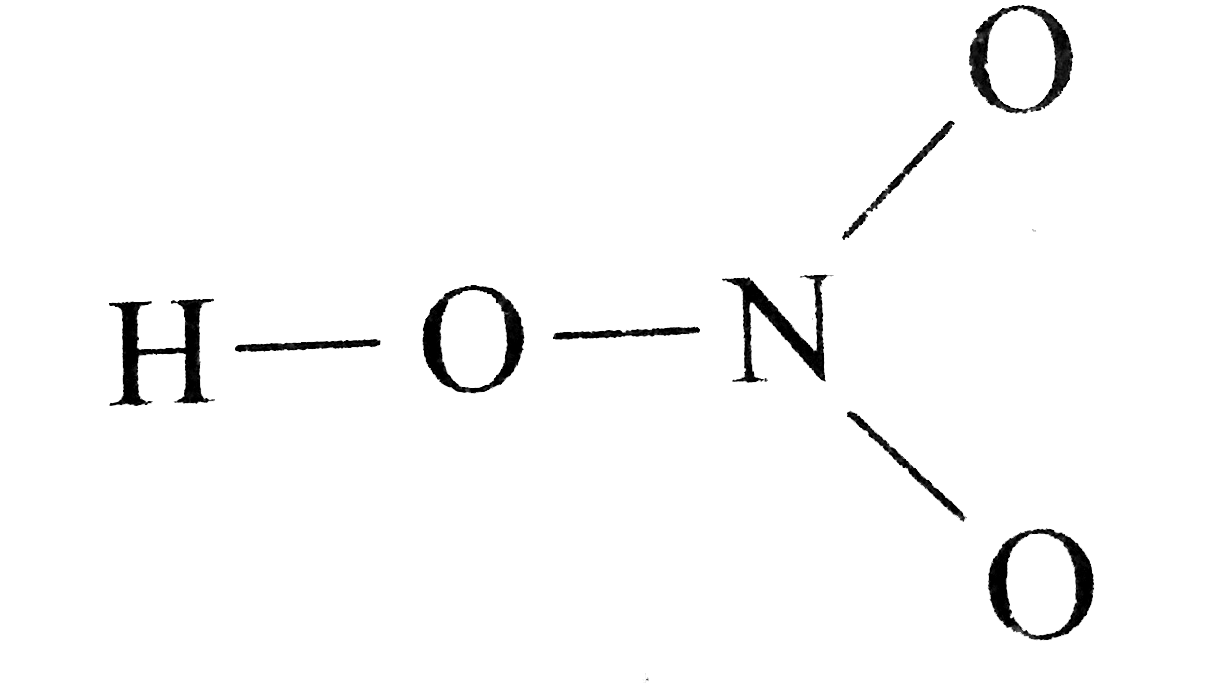


Draw The Lewis Structure Of Nitric Acid Hno 3
See Answer Check out a sample Q&A hereLearn this topic by watching Electron Geometry Concept VideosProblem Draw the Lewis structure for hydrogen peroxide, H2O2 FREE Expert Solution Total number of valence electrons Group Valence Electrons H 1A 2 × 1 e – = 2 e – O 6A 2



The Shape Of The Hydrogen Peroxide Molecule In The Gas Phase Gas Molecules Tech Company Logos


Potassium Peroxide K2o2 Chemspider
Hydrogen peroxide (H 2 O 2, HOOH) The simplest peroxide Molecular Structure of Hydrogen Peroxide Lewis structure Ball and spoke Spacefilling model Molecular model kit Related terms Hydroperoxide, hydroperoxyl radical, peracid, mCPBALewis structure of Hydrogen peroxide (H 2 O 2) contains two OH bonds and one OO bond Also, there are two lone pairs on each oxygen atom Also, there are two lone pairs on each oxygen atom Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of H 2 O 2Structural Formula O 2 2 Peroxide Ion



Chapter 8 Concepts Of Chemical Bonding Ppt Download


Lewis Structure Of H2o2
Oct 23, · Draw the Lewis structure for a peroxide (0,) ion C ?70 More Lewis Dot Structures Element number 8 and a member of the Chalcogen Familyor Group 16 of the periodic table From http//enwikipediaorg/wiki/PeroxideThe peroxide ion contains two electrons more than the oxygen molecule These two electrons, according to the molecular orbital theory, complete the two π* antibonding orbitalsNov 11, 17 · The Lewis structure shows an arrangement of how the elements are connected to each other within a compound using electron dots or bonds They follow the octet rule, wherein each element must be surrounded by 8 electrons Each single bond consists of 2 electrons An exception to this rule is the hydrogen atom



Hooh Lewis Structure How To Draw The Lewis Structure For Hydrogen Peroxide Youtube



H2o2 Lewis Structure Hydrogen Peroxide Molecular Geometry Polarity
May 29, 21 · Hydrogen Peroxide consists of 2 hydrogens and 2 oxygen atoms arranged in an open book like structure with bent OHO bonds The electronegativity of oxygen is around 344 and that of hydrogen is 22 The difference between the electronegativity of O and H atoms causes the OH bond to be polarLet's do the Lewis structure for H2O2 Hydrogen Peroxide, also called dihydrogen dioxide On the periodic table, Hydrogen's in group 1 so it has 1 valence electron, but we have two of them, so we need to multiply by 2 Oxygen, group 6 or 16, we have two of those, so let's multiply that by 2 as well for a total of 14 valence electronsStructure and properties In O 2 F 2, oxygen is assigned the unusual oxidation state of 1 In most of its other compounds, oxygen has an oxidation state of −2 The structure of dioxygen difluoride resembles that of hydrogen peroxide, H 2 O 2, in its large dihedral angle, which approaches 90° and C 2 symmetry



Zinc Peroxide H2o2zn Pubchem



Write The Lewis Structure Of Hydrogen Peroxide
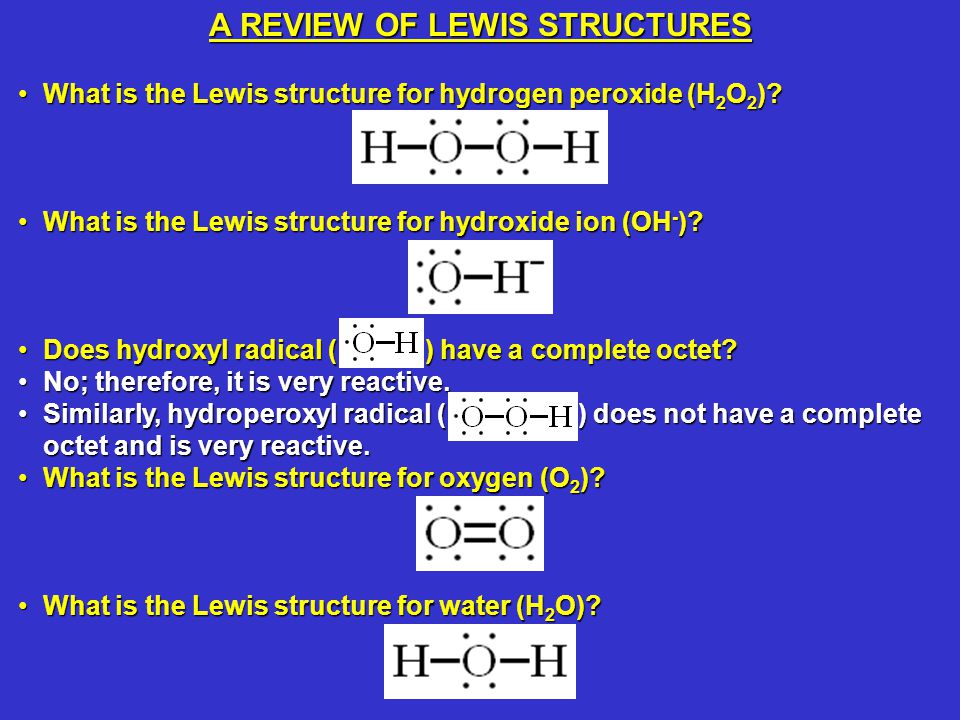
Jul , 13 · A stepbystep explanation of how to draw the O2 2 Lewis Dot Structure (Peroxide Ion)For the O2 2 structure use the periodic table to find the total numbeHydrogen peroxide, H2O2, the oxygen atoms are in the center (H–O–O–H) 7 In drawing Lewis structures for relatively small molecules and polyatomic ions, the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms EXAMPLE Write the Lewis structure for CH2O where carbon is the central atomHydrogen peroxide is a colorless liquid at room temperature with a bitter taste Small amounts of gaseous hydrogen peroxide occur naturally in the air Hydrogen peroxide is unstable, decomposing readily to oxygen and water with release of heat Although nonflammable, it is a powerful oxidizing agent that can cause spontaneous combustion when it


O2 2 Peroxide Ion Lewis Structure


Redox Reactions
Dec 24, 15 · $\ce{S=O}$ has a bond energy of around $\pu{128 kcal/mol}$, but $\ce{OO}$ has a measly bond energy of $\pu{35 kcal/mol}$ So, in future, if you are asked to draw Lewis structure for compounds, avoid using multiple peroxide linkages There can be a maximum of one* in your structureWrite the Lewis structure for the following compound Hydrogen peroxide ({eq}H_2O_2 {/eq})Fullscreen check_circle Expert Answer Want to see the stepbystep answer?


Peroxide Wikipedia



Reduction Of O2 To H2o And Its Free Radical Intermediates A Lewis Download Scientific Diagram
Switch to Home Your dashboard and recommendations Booster Classes Personalized courses, with or without credits Homework Help What is the Lewis structure for the molecule hydrogen peroxide, H2O2?Lewis structure of oxygen Preparation of Oxygen a Thermal decomposition of potassium chlorate(V) 2 KClO3 ( s ) 2 KCl ( s ) 3O2 ( g ) → Drying agent CaCl2, CaO, concentrated H2SO4 heat ,300ο C MnO2 b Catalytic decomposition of hydrogen peroxide solution 2 H 2O2 (aq) 2 H 2O(l ) O2 ( g ) →Jan 21, 19 · Let's do the Lewis structure for H2O2 Hydrogen Peroxide, also called dihydrogen dioxide On the periodic table, Hydrogen's in group 1 so it has 1 valence electron, but we have two of them, so we need to multiply by 2 What is the Lewis structure for H 2 O 2?
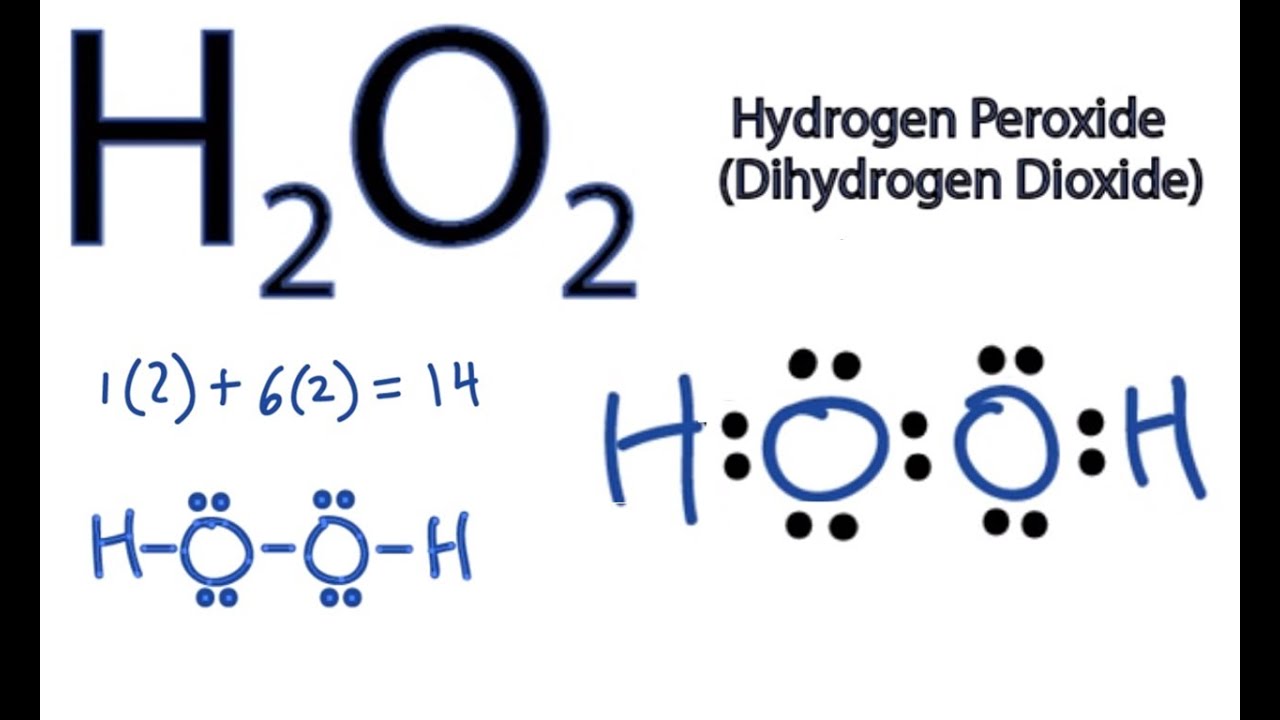


H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Chemical Bonding Success In Chemistry
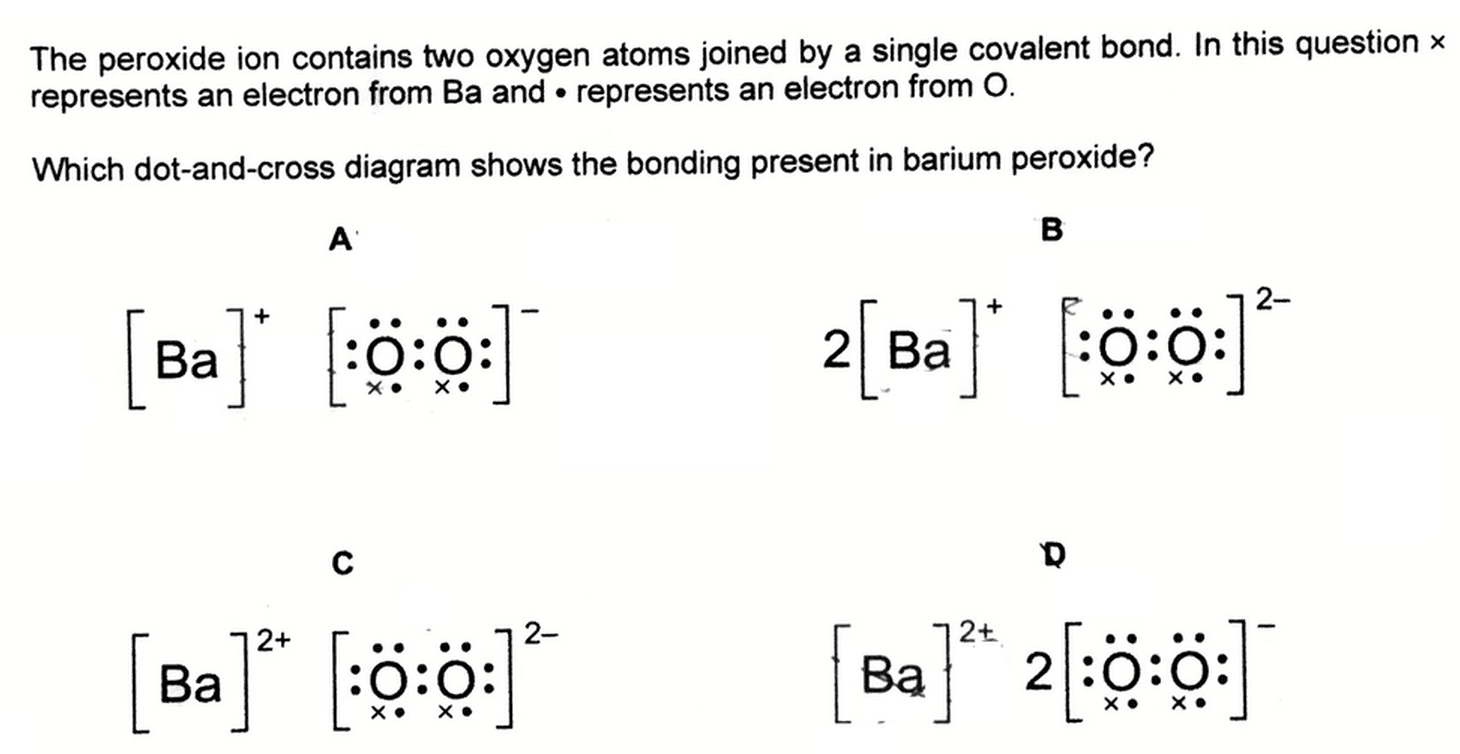


Barium Peroxide Dot Cross Diagram
Dec 11, 15 · Trying to figure out where peroxide gains 2 electrons and why it has a 2 charge?Draw the lewis structure of Cl 2 O 2 based on this structure ClCl with the 2 Oxygen's branching off of the Chlorine Do not add formal charges Then, what is the geometry about the central chlorine atom?Sep 14, 18 · A stepbystep explanation of how to draw the O2 2 Lewis Structure(Peroxide Ion) For the O2 2 Lewis structure, calculate the total number of valence electrons for the O2 2 molecule After determining how many valence electrons there are in O2 2, place them around the central atom to complete the octets


Magnesium Peroxide Mgo2 Chemspider


Sodium Peroxide Na2o2 Pubchem
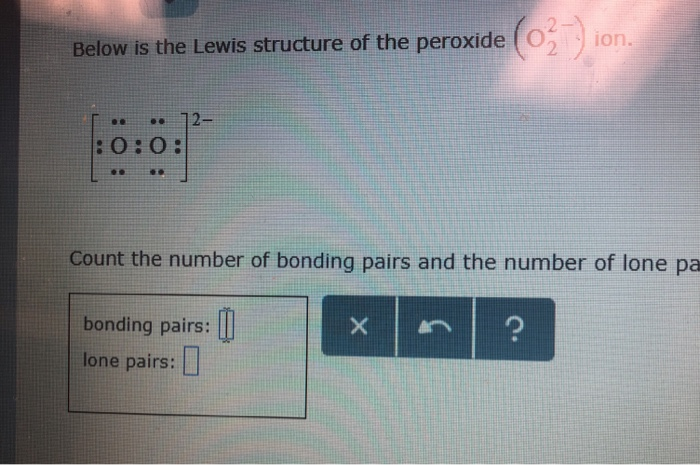
Below is the Lewis structure of the peroxide 0 ion 12 00 Count the number of bonding pairs and the number of lone pairs around the right oxygen atom bonding pairs lone pairsLewis structures are preferable when adjacent formal charges are zero or of the opposite sign Toothpastes containing sodium hydrogen carbonate (sodium bicarbonate) and hydrogen peroxide are widely used Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriateA peroxide is any compound which has two oxygen atoms bonded together The OO group is the peroxide group of the compound And Hydrogen Peroxide is the simplest peroxide The chemical formula for hydrogen peroxide is H2O2 It is a water molecule with one extra atom of oxygen, It has various uses ranging from disinfectant to propellant for



Hydrogen Peroxide Molecule And Its Lewis Dot Electron Dot Structure H2o2 Formal Science Chemistry Essay Essaysusa Com



Is H2o2 Polar Or Nonpolar Techiescientist
Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO −It is an unstable structural isomer of nitrate, NO − 3Although its conjugate acid peroxynitrous acid is highly reactive, peroxynitrite is stable in basic solutions It is prepared by the reaction of hydrogen peroxide with nitrite H 2 O 2 NO − 2 → ONOO − H 2 OStructure, properties, spectra, suppliers and links for Potassium peroxide, K2O28 rows · According to the Hydrogen peroxide lewis structure, it contains a total of 4 lone pairs and each


Answered A Construct A Lewis Structure For Bartleby


Benzoyl Peroxide Cas 94 36 0 Chemsrc
(a) Draw the Lewis structure for hydrogen peroxide, H 2 O 2 (b) What is the weakest bond in hydrogen peroxide?(c) Hydrogen peroxide is sold commercially as an aqueous solution in brown bottles to protect it from lightCalculate the longest wavelength of light that has sufficient energy to break the weakest bond in hydrogen peroxideJan 05, 21 · Get the detailed answer What is the Lewis structure for the molecule hydrogen peroxide, H2O2?Probing secondary coordination sphere influence on the oxygenation of zinc alkyls formation of a unique zinc peroxide species Chemical Communications 13, 49 () , DOI /c3cc466d Yan Liu, Yong Cai Zhang, Ming Zhang



Learn Properties And Structure Of Hydrogen Peroxide In 3 Minutes


Chem4kids Com Hydrogen Orbitals And Compounds
Sep 10, 18 · Benzoyl peroxide exists as a colourless solid which has a crystalline structure It has a faint odour which resembles the smell of benzaldehyde C 14 H 10 O 4 is insoluble in water, somewhat soluble in alcohols, and quite soluble in ethers and chloroformHydrogen Peroxide The chemical name for H 2 O 2 is hydrogen peroxideFeb 25, 11 · Hydrogen peroxide has a bent shape and is a polar molecule It may seem odd that hydrogen peroxide has a bent shape When the Lewis structure is drawn it looks similar to this HOOH , with two unshared pairs on each of the oxygen This may appear to be a linear shaped molecule, but after further research this is not the case


Barium Peroxide Bao2 Chemspider



4 General Organic And Biochemistry 8 E Bettelheim



How Many Nonbonding Electron Pairs Are The Clutch Prep
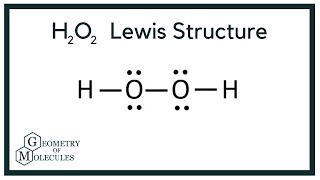


H2o2 Lewis Structure Hydrogen Peroxide Youtube



Write The Lewis Structure Of Hydrogen Peroxide Youtube


Hydrogen Peroxide H2o2 Pubchem



Lewis Dot Structure Of O2 2 Peroxide Ion Youtube


Lewis Structure High Res Stock Images Shutterstock



H2o2 Lewis Structure Shape Novocom Top


Lewis Structure Hydrogen Peroxide Molecule Structural Formula Png Clipart Angle Brand Chemical Bond Chemical Compound Chemical



Solved 19 A Student Proposed The Lewis Structure To The Chegg Com



H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Molecular Geometry Molecular Shapes Intermolecular Force



What Is The Lewis Structure For H2o2 Study Com


What Is The Electron Geometry Of H2o2
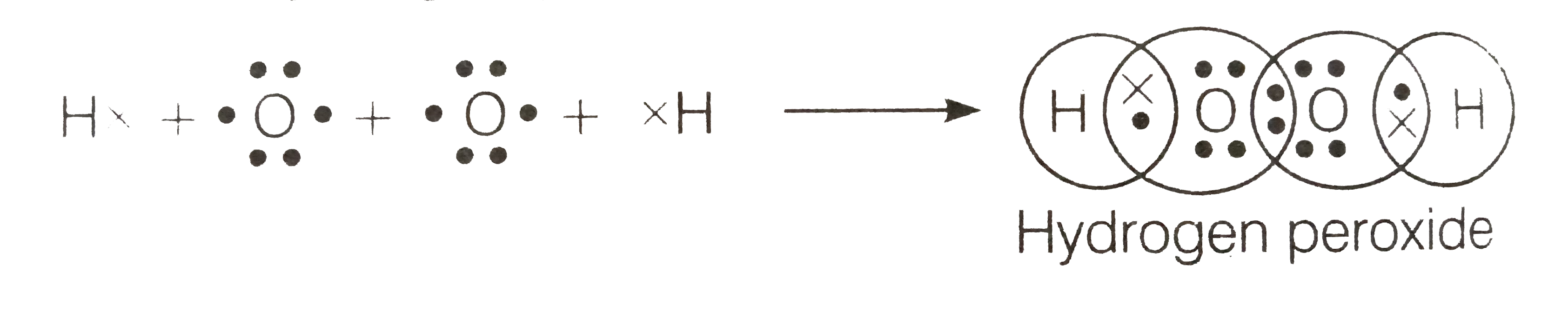


Write The Lewis Structure Of Hydrogen Peroxide



Calcium Peroxide Cas No 1305 79 9 Knowledge Echemi



Chromium Vi Oxide Peroxide Wikipedia


Ch 103 Percent Hydrogen Peroxide Ppt Video Online Download



Draw The Lewis Structure For Hydrogen Pero Clutch Prep
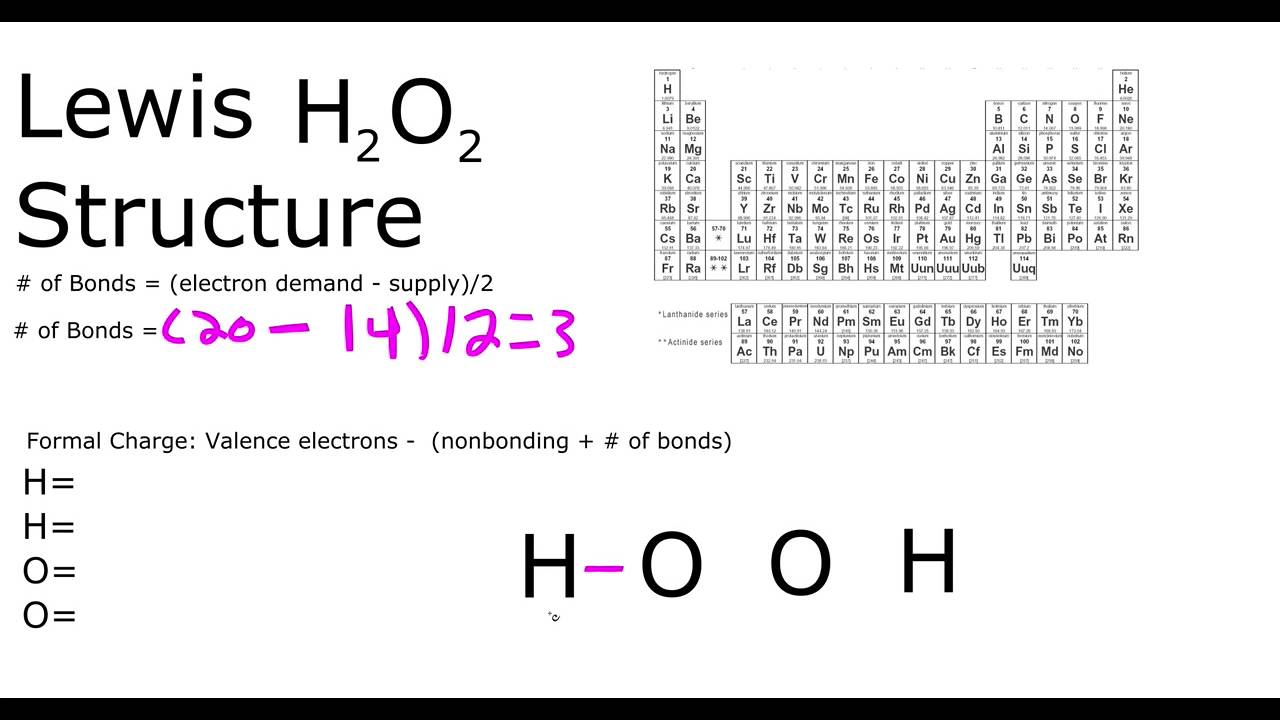


H2o2 Lewis Structure Youtube



Solved 1 Write Lewis Structures For Each Of These Compou Chegg Com


H2o2 Lewis Structure



Draw A Lewis Dot Structure Of D2o2 Novocom Top



H2 Lewis Structure Novocom Top



Lewis Structure Hydrogen Peroxide Molecule Structural Formula Laughing Gas Structure Angle Text Png Pngegg


Chlorine Peroxide Cl2o2 Chemspider
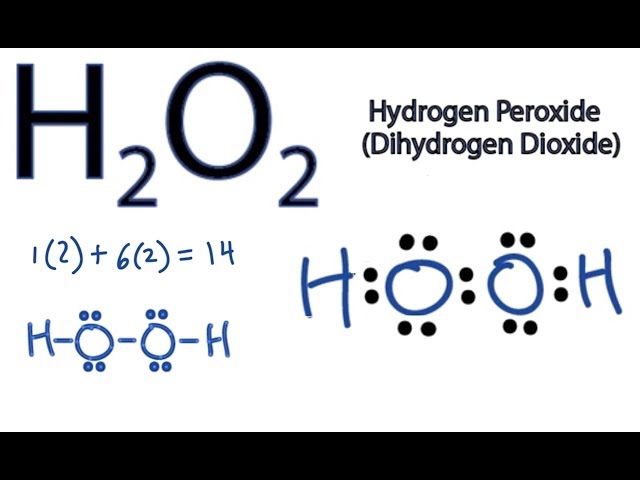


H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Youtube
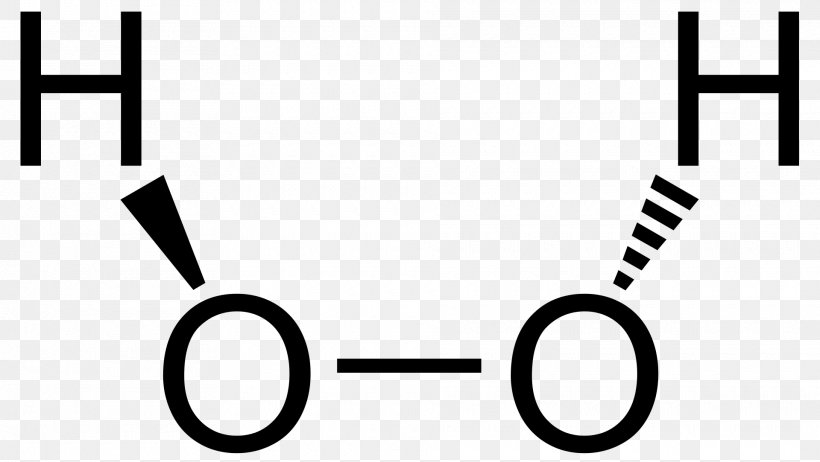


Hydrogen Peroxide Lewis Structure Chemistry Barium Peroxide Png 19x10px Hydrogen Peroxide Area Atom Barium Peroxide Black


Hydrogen Peroxide Franzcalvo
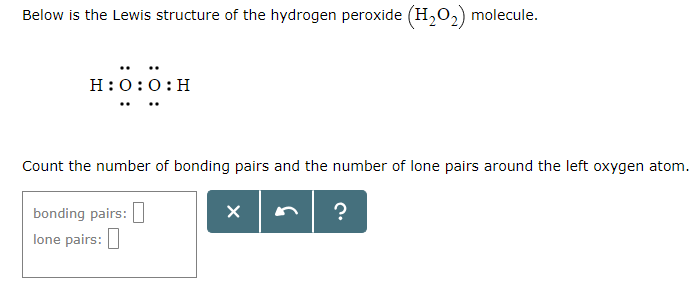


Solved Below Is The Lewis Structure Of The Hydrogen Perox Chegg Com



Lewis Structures Simple Organic Compounds Janet Gray Coonce


7 E Chemical Bonding And Molecular Geometry Exercises Chemistry Libretexts



Hydrogen Peroxide H2o2 Lewis Structure Novocom Top



The Lewis Structure For H2o2 Novocom Top
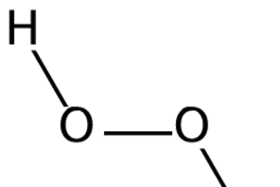


Hydrogen Peroxide Soundbite Rsc Education



Download Hydrogen Peroxide Structure Clipart Lewis Structure Full Size Png Image Pngkit
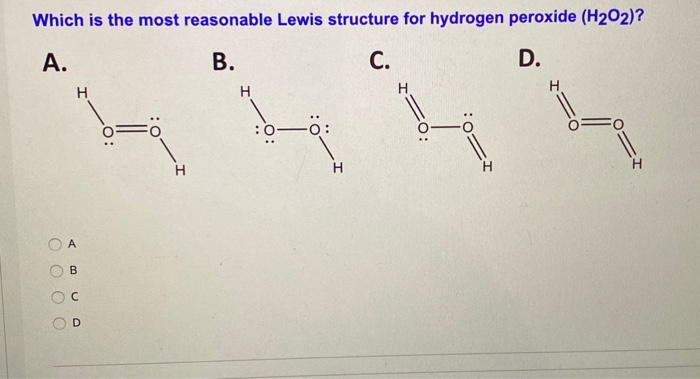


Solved Which Is The Most Reasonable Lewis Structure For H Chegg Com
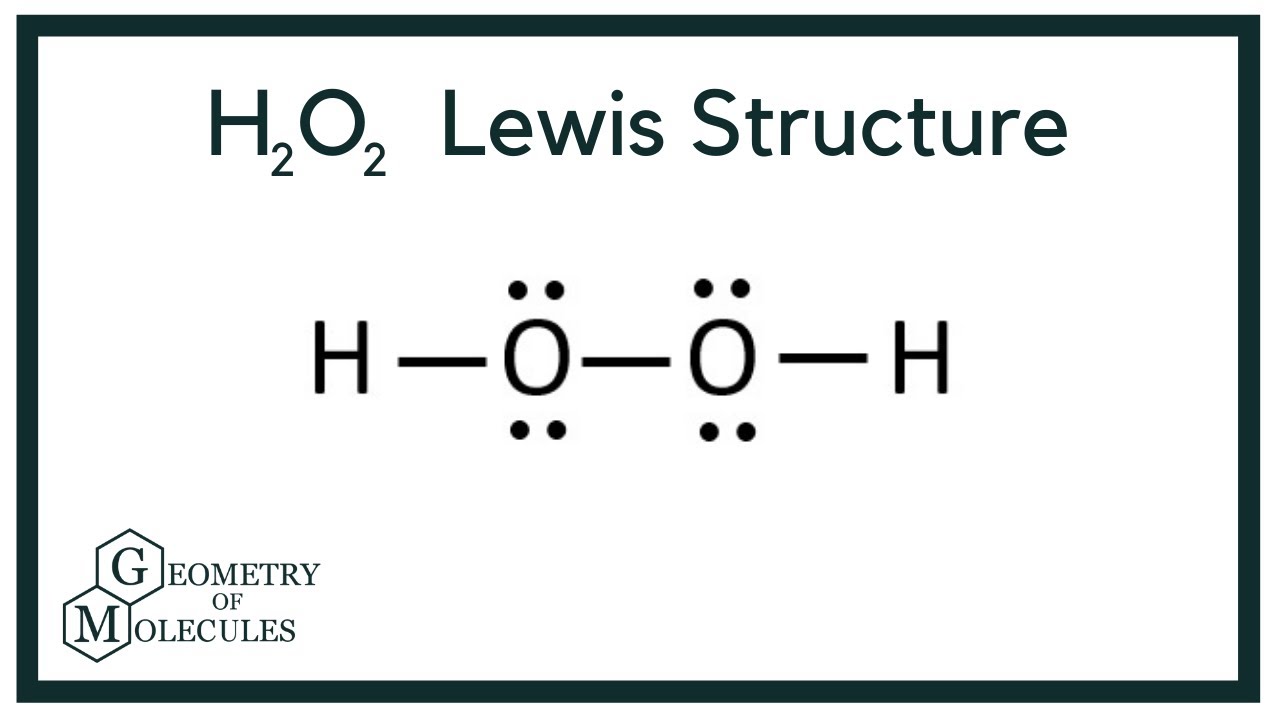


H2o2 Lewis Structure Hydrogen Peroxide Youtube



Hydrogen Peroxide Lewis Dot Novocom Top



Oneclass Draw The Lewis Electron Dot Structure For Hydrogen Peroxide Which Is Used To Bleach Hair



Lewis Structures Simple Organic Compounds Janet Gray Coonce



Hydrazine Nh2 Nh2 Hydrogen Peroxide H Clutch Prep



Mo 0 2 Drawing A Lewis Structure Of Oxygen Superoxide Peroxide Youtube
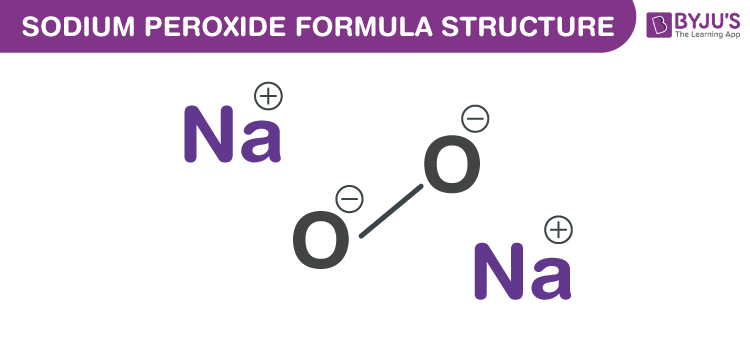


Sodium Peroxide Formula Chemical Formula Structure And Properties



Hydrogen Peroxide Molecule Chemical Compound Lewis Structure Decomposition Hydrogen Catalysis Png Pngegg
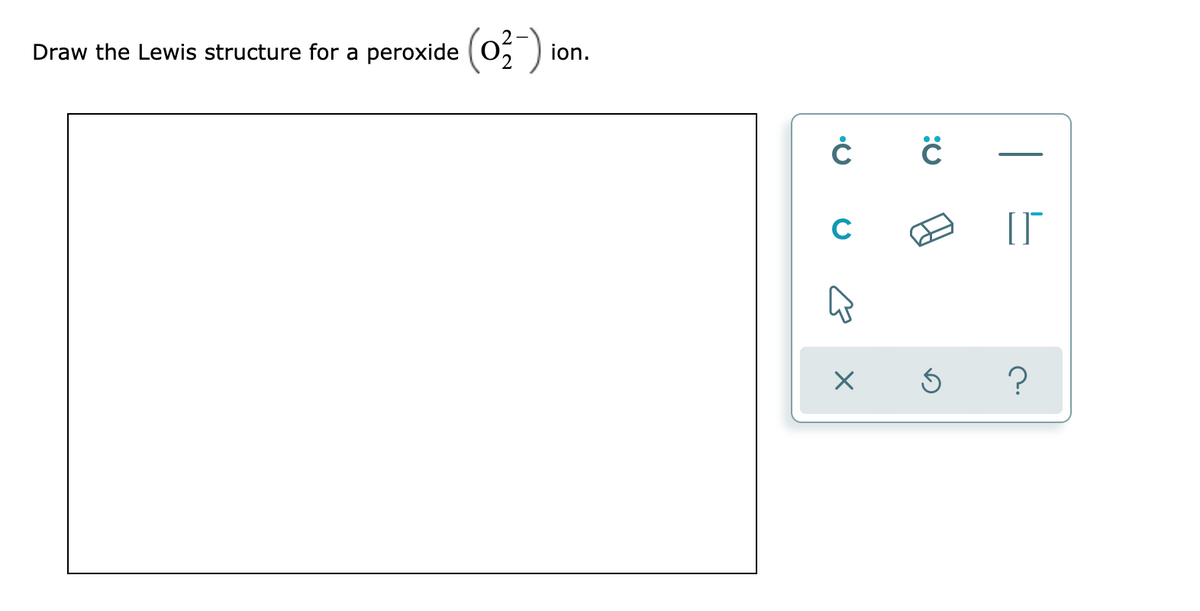


Answered Draw The Lewis Structure For A Peroxide Bartleby



Hydrogen Peroxide



Peroxide And 2 Extra Electrons Chemistry Stack Exchange



Peroxide


Hydrogen Peroxide Reactions And Physical Properties H2o2



Learn Properties And Structure Of Hydrogen Peroxide In 3 Minutes



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Royalty Free Cliparts Vectors And Stock Illustration Image


Is H2o2 Polar Or Non Polar Quora



What Vsepr Shape Is H2o2
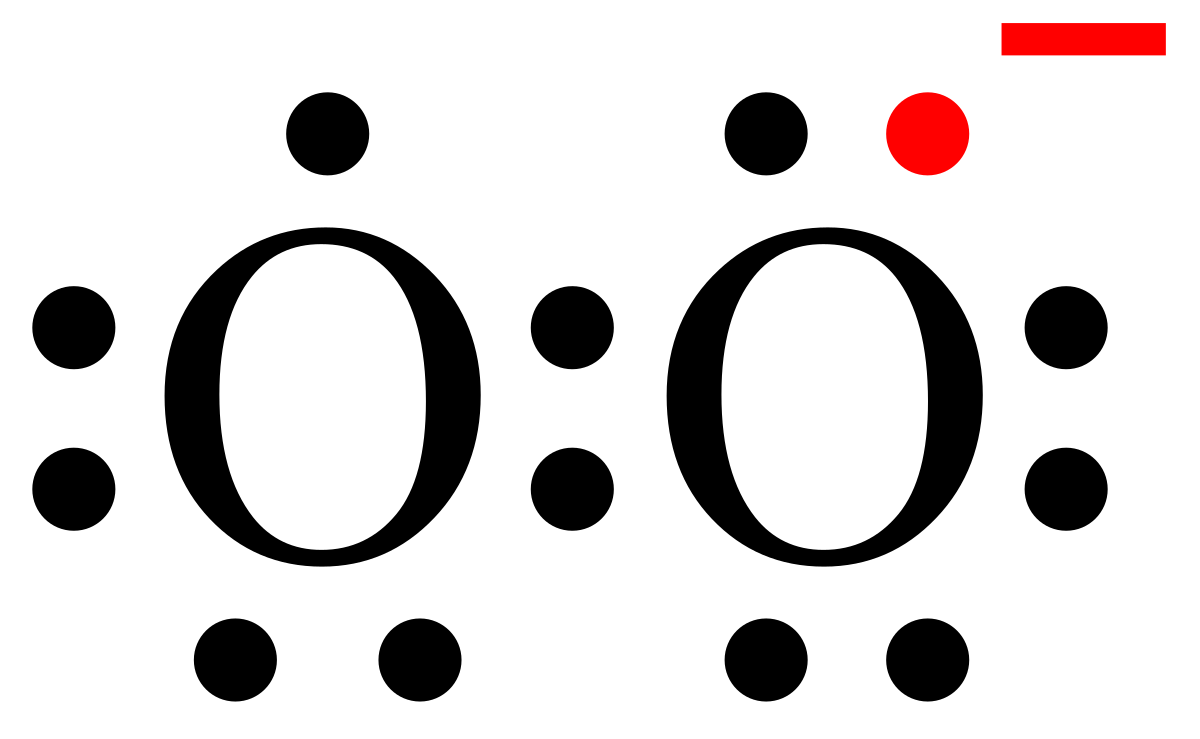


Superoxide Wikipedia
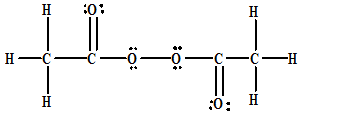


An Objectionable Component Of Smog Is Acetyl Peroxide Which Has The Following Skeleton Structure A Draw The Lewis Structure Of This Compound B Write The Bond Angles Indicated By The Numbered Angles



Below Is The Lewis Structure Of The Peroxide O2 2 Ion Co Clutch Prep
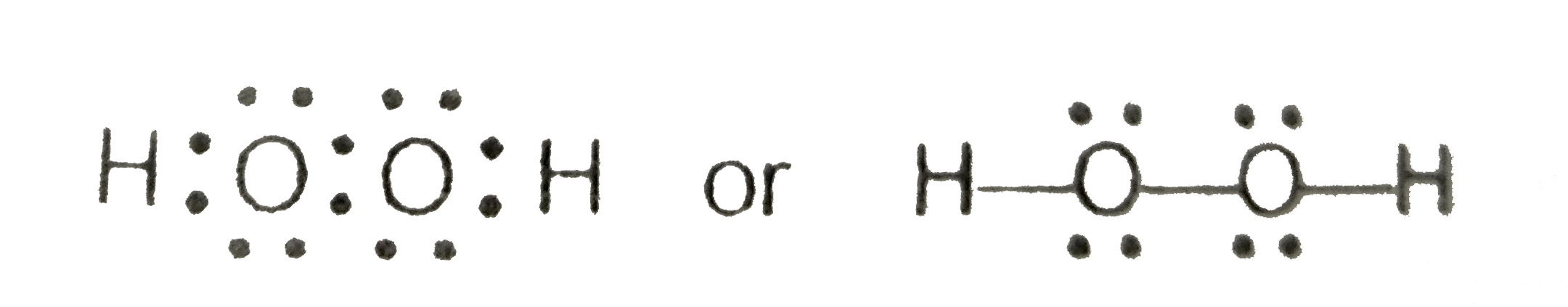


Write The Lewis Structure Of Hydrogen Peroxide
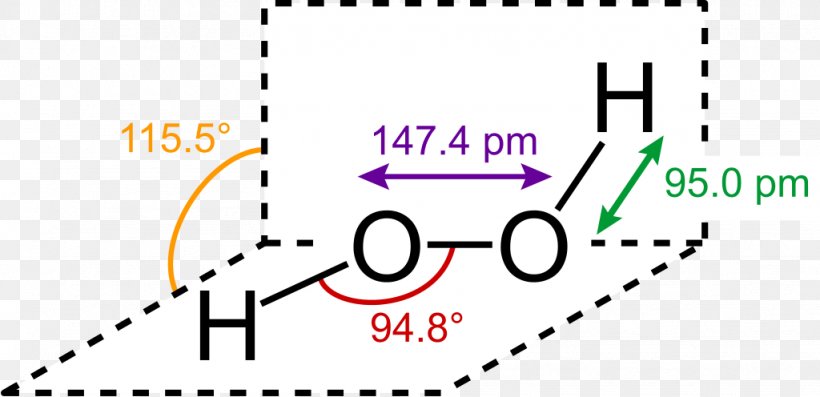


Lewis Structure Hydrogen Peroxide Molecule Structural Formula Png 1024x496px Lewis Structure Area Brand Chemical Bond Chemical
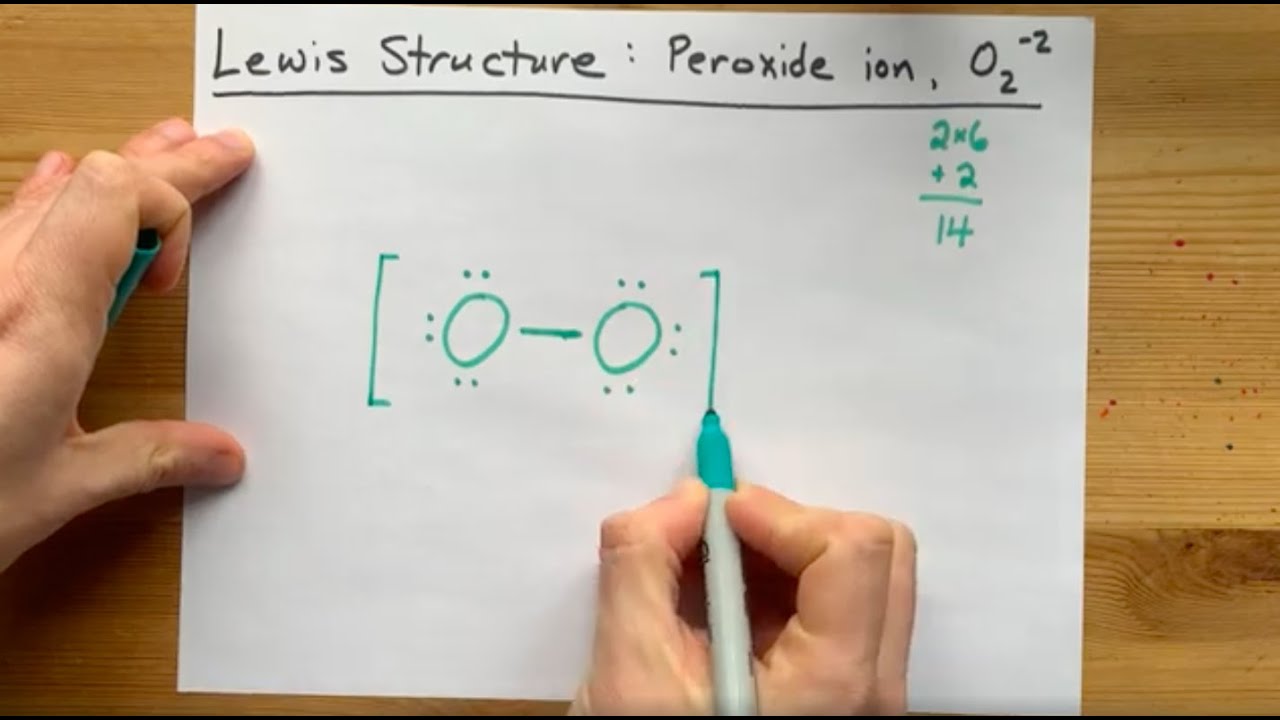


Lewis Structure Of The Peroxide Ion O2 2 Youtube
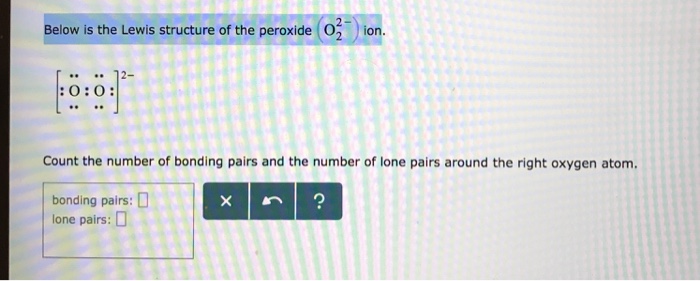


Solved Below Is The Lewis Structure Of The Peroxide 0 Ion Chegg Com
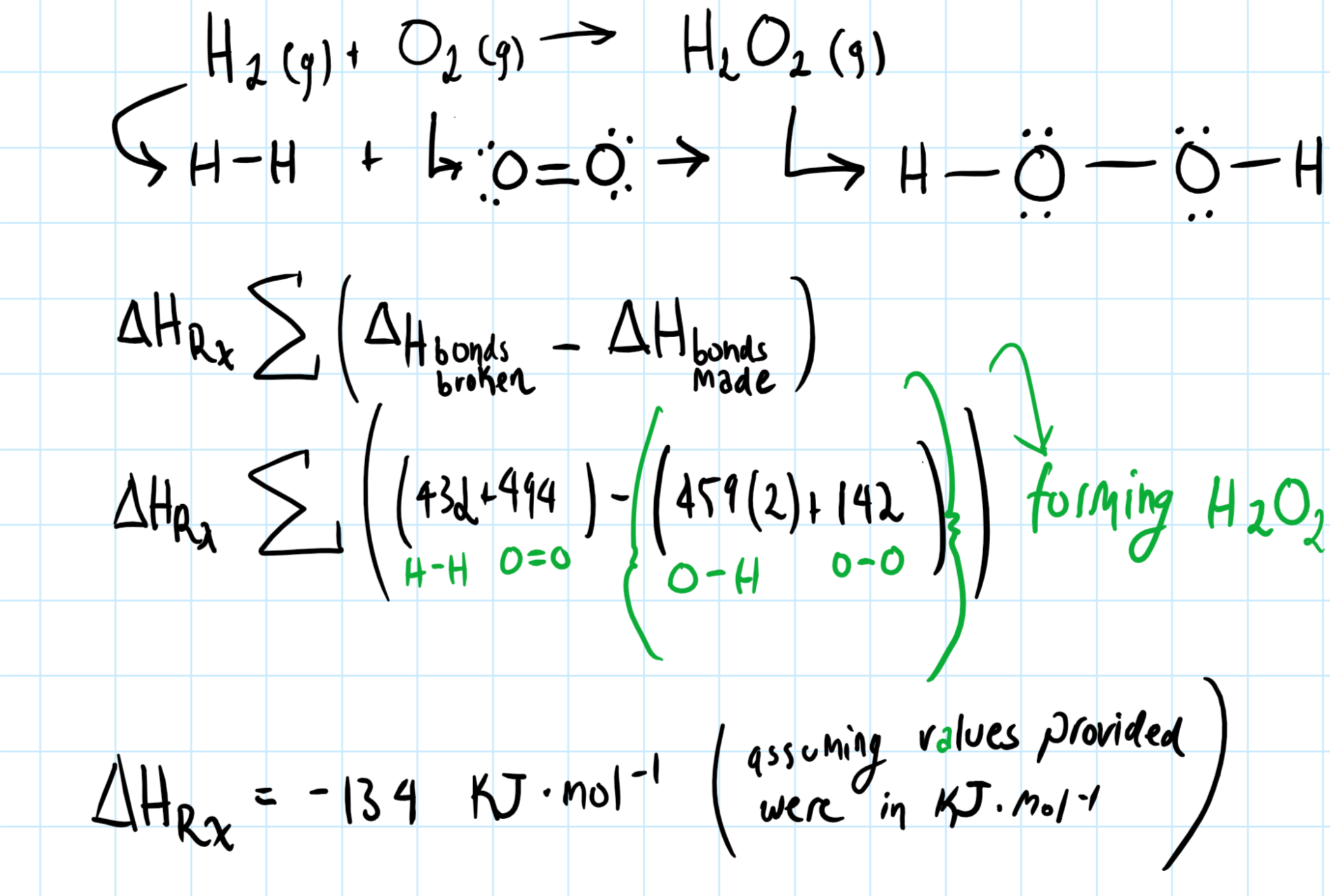


How Do You Determine The Heat Of Formation Of Hydrogen Peroxide From The Given Bond Energies H 2 G O 2 G H 2o 2 G Bond Energies H H 432 O2 494 O H 459 O O 142 Socratic


Sodium Peroxide Na2o2 Chemspider



Write The Lewis Structure Of Hydrogen Peroxide
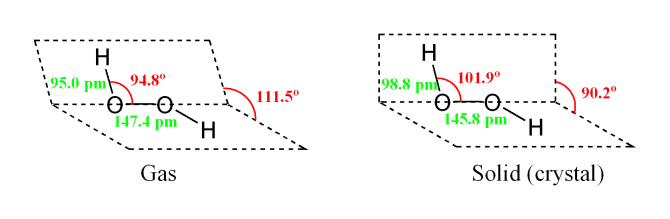


File H2o2 Structure Png Wikimedia Commons



Lewis Dot Structure Of O2 2 Brainly In



Video Lewis Structure For H2o2



Construct A Lewis Structure For Hydrogen Peroxide H2o2 In Which Each Atom Achieves An Octet Of Electrons


Barium Peroxide Bao2 Pubchem



O2 2 Lewis Structure How To Draw The Lewis Structure For O2 2 Youtube



Food Grade Hydrogen Peroxide Food Grade Hydrogen Peroxide Hydrogen Peroxide Food Grade



Solved Below Is The Lewis Structure Of The Peroxide 03 Chegg Com



Lewis Structure Of Hydrogen Peroxide H2o2 Novocom Top


Lewis Structure Of Sodium Peroxide Cad Vigyan



How Many Bonding Electrons Are Between The Clutch Prep


Solved Below Is The Lewis Structure Of The Peroxide O Io Chegg Com



0 件のコメント:
コメントを投稿